Tumor Infiltrating Lymphocyte (TIL) Cell Therapy in Melanoma
This slide show is not intended to be a substitute for professional medical advice. Always consult your doctor about any questions you may have regarding a medical condition.
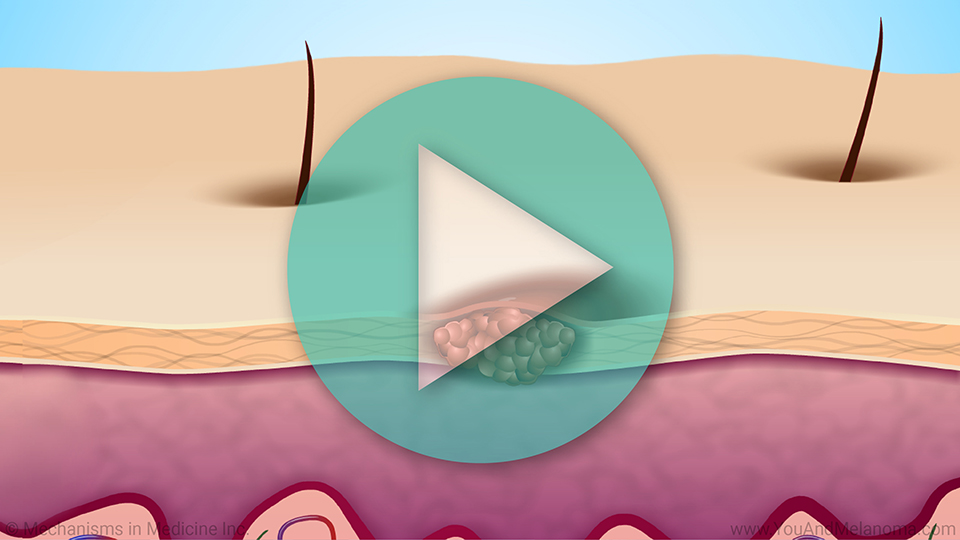
Your immune system naturally produces T cells
When cancer cells first appear in your body, your immune system naturally produces a specific type of immune cell, called a T-cell, that can recognize and attack tumors.1
This is part of your natural immune response.
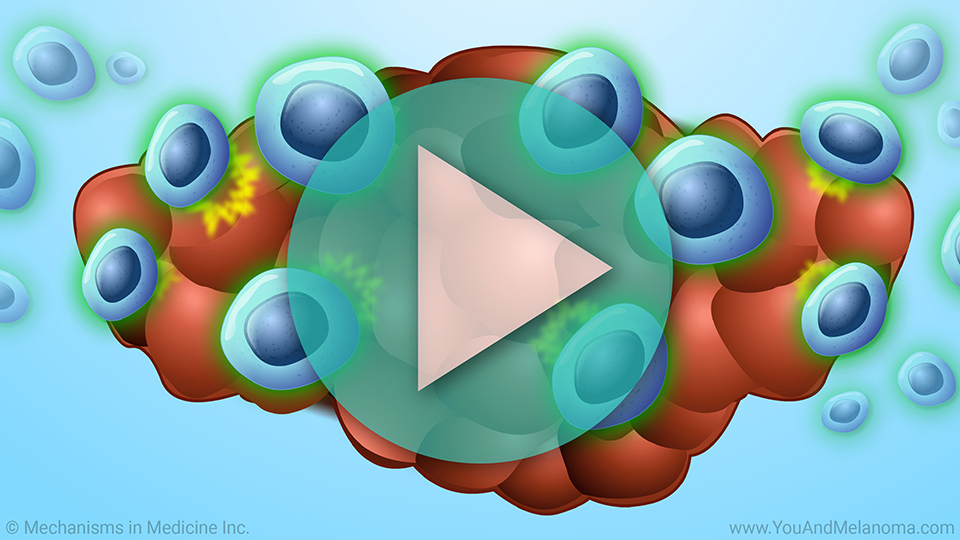
T cells infiltrate the tumor to fight cancer
When T-cells infiltrate (go inside) your tumor, they are called tumor infiltrating lymphocytes or TILs.1
TILs can naturally fight cancer cells.1
Cancer evades the natural immune response
Unfortunately, cancer cells eventually find ways to evade TILs and can overpower your body's immune defenses. When this happens, your natural TILs may become ineffective or there may be too few in number to fight the cancer.2
What is TIL Cell Therapy?
TIL Cell Therapy or Tumor Infiltrating Lymphocyte Cell Therapy is a new type of immunotherapy that utilizes TILs to attack cancer cells.1
TIL Cell Therapy is investigational and only available in clinical trials for solid tumors like melanoma; it is not yet approved by the FDA.
How does TIL Cell Therapy work?
TIL Cell Therapy works by adding billions of tumor infiltrating lymphocytes (TILs) to your body that were made in the lab and can destroy cancer cells.1
How does TIL Cell Therapy work?
In TIL Cell Therapy, TILs circulate in your bloodstream and travel into a tumor where they can recognize specific tumor-associated antigens.
How does TIL Cell Therapy work?
TIL Cell Therapy delivers TILs that are cytotoxic to the cancer cells and destroy the tumor.
Preparing TIL Cell Therapy in the Lab
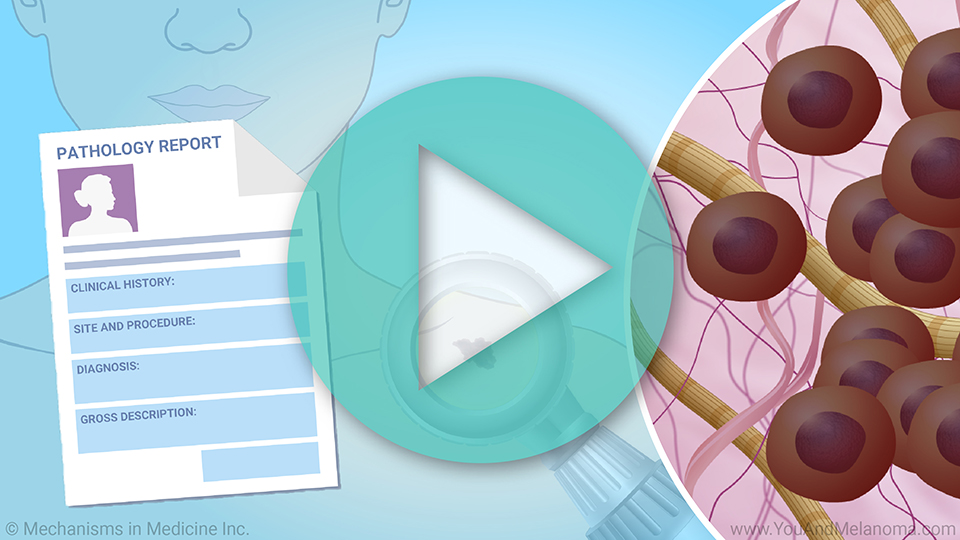
To make TIL Cell Therapy, doctors first take a sample of your melanoma tumor and send it to the manufacturing lab.
Preparing TIL Cell Therapy in the Lab
The TILs are removed from the tumor sample.
Preparing TIL Cell Therapy in the Lab
The TILs are grown in tissue culture with specific growth factors (rIL-2) and feeder cells.
The TILs multiply in numbers upwards of a hundred billion cells.
Preparing TIL Cell Therapy in the Lab
Following the manufacturing process the TILs are removed from the tissue culture where they were growing and prepared for IV administration.
Manufacturing takes several weeks
This process takes several weeks to prepare in the lab. Some lab processes have shortened manufacturing to 3 weeks or less.
Receiving chemotherapy while TIL Cell Therapy is being made
While the TILs are being reproduced in the lab, to prepare your body to accept the TIL infusion, you will first receive a short course of chemotherapy to prepare your immune system.
This reduces the number of your body’s existing immune cells.
Administering TIL Cell Therapy
Next, you will receive the infusion of the TIL Cell Therapy that was generated in the lab personally for you.
You will be hospitalized during this entire process so that you can be closely monitored for any adverse side effects.
Circulating TILs can attack tumor cells
Once reinfused back into you, TILs can effectively recognize and attack the tumor cells.1
TILs can increase more than 1000-times after IV infusion
TILs can increase more than 1000-times after IV infusion, and they can continue to replicate over time in your body.3
Administration of IL-2 following TIL Cell Therapy
Administration of a short course of IL-2 following the TIL Cell Therapy infusion promotes activation, proliferation, and tumor-killing activity of the TIL.
Patients participating in clinical trials of TIL Cell Therapy may receive many doses of IL-2 to help the TILs multiply and work against the cancer.4
Side effects of TIL Cell Therapy
While there can be side effects of TIL Cell Therapy, such as fever, chills, diarrhea, nausea, vomiting, and low counts of blood cells – both red blood cells and white blood cells – as well as platelets, side effects are generally short in duration and resolve within the first few weeks.5
Limits of TIL Cell Therapy
For someone to receive TIL Cell Therapy, their cancer must be stable enough to wait several weeks while TILs are prepared in the manufacturing lab.3
Patients must also be healthy enough to have chemotherapy before TIL Cell Therapy.
When is TIL Cell Therapy used?
TIL Cell Therapy is being investigated in clinical trials for advanced melanoma that is inoperable or has spread or metastasized to other parts of the body from where it originated.6 TIL Cell Therapy may be used in metastatic melanoma of the skin, eye, and mucus membranes of the body.
When is TIL Cell Therapy used?
TIL Cell Therapy is also being investigated in other solid tumors and hematologic malignancies in clinical trials when all approved therapies have failed.7
TIL Cell Therapy after other treatments for melanoma
In advanced melanoma patients, immune checkpoint inhibitors or targeted therapy may have been tried and not been successful at elimination of all tumors.4
Some studies are assessing the use of TIL Cell Therapy at an earlier stage in melanoma treatment.
How effective is TIL Cell Therapy for metastatic melanoma?
In clinical trials, TIL Cell Therapy has shown promising results in facilitating tumor regression.3,4
TIL Cell Therapy has clear advantages in the treatment of solid tumors as it is personalized with autologous (self-derived) TIL which utilizes self-presentation molecules and recognizes the broad expression of a patient’s tumor-specific antigens.
TIL Cell Therapy is a one-time treatment
In clinical trials, TIL Cell Therapy is a one-time treatment with no maintenance therapy.
Patients treated in clinical trials at the National Cancer Institute (NCI)
In patients treated in clinical trials at the National Cancer Institute (NCI), 40-60% of advanced melanoma patients were able to achieve some level of response.
About 20% of patients had complete response
About 20% of patients in this study had complete response rates.8
Complete response is determined when melanoma is no longer seen on body scans.
Follow-up studies at 3-5 years
In follow-up studies at 3 to 5 years, most of the patients who initially achieved complete response stayed in complete response.8
These are very promising numbers in patients who have failed other therapies.
Survival after TIL Cell Therapy
So far, TIL Cell Therapy has only been available in clinical trials, because its use is still under investigation, and it has not yet been approved by the US FDA.
Survival after TIL Cell Therapy
Patients receiving TIL Cell Therapy have survived longer – sometimes years longer – than expected with melanoma that has failed 1st line therapy.
In the future, TIL Cell Therapy may be an important option for treating melanoma and other cancers.
Questions for your doctor about TIL Cell Therapy
In the meantime, you may want to ask your doctor if a clinical trial with TIL Cell Therapy could be an option for you.
References
-
Badalamenti G, Fanale D, Incorvaia L, Barraco N, Listì A, Maragliano R, Vincenzi B, Calò V, Iovanna JL, Bazan V, Russo A. Role of tumor-infiltrating lymphocytes in patients with solid tumors: Can a drop dig a stone? Cell Immunol. 2019;343:103753. doi: 10.1016/j.cellimm.2018.01.013.
-
Monjazeb AM, Zamora AE, Grossenbacher SK, Mirsoian A, Sckisel GD, Murphy WJ. Immunoediting and antigen loss: overcoming the achilles heel of immunotherapy with antigen non-specific therapies. Front Oncol. 2013;3:197. doi: 10.3389/fonc.2013.00197.
-
Rosenberg SA, Restifo N. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348(6230):62-68. doi: 10.1126/science.aaa4967.
-
Sarnaik AA, Hamid O, Khushalani NI, Lewis KD, Medina T, Kluger HM, Thomas SS, Domingo-Musibay E, Pavlick AC, Whitman ED, Martin-Algarra S, Corrie P, Curti BD, Oláh J, Lutzky J, Weber JS, Larkin JMG, Shi W, Takamura T, Jagasia M, Qin H, Wu X, Chartier C, Graf Finckenstein F, Fardis M, Kirkwood JM, Chesney JA. Lifileucel, a tumor-infiltrating lymphocyte therapy, in metastatic melanoma [published correction appears in J Clin Oncol. 2021;39(26):2972]. J Clin Oncol. 2021;39(24):2656-2666. doi: 10.1200/JCO.21.00612.
-
Wolf B, Zimmermann S, Arber C, Irving M, Trueb L, Coukos G. Safety and Tolerability of Adoptive Cell Therapy in Cancer. Drug Saf. 2019;42:315-334. doi: 10.1007/s40264-018-0779-3.
-
Melanoma Research Alliance. Interleukin-2. Updated August 2021. Available at:
https://www.curemelanoma.org/patient-eng/melanoma-treatment/immunotherapy/interleukin-2-il-2-proleukin/. Accessed September 8, 2021.
-
Fardis M, DiTrapani K, Chartier C, Graf Finckenstein F. Current and future directions for tumor infiltrating lymphocyte therapy for the treatment of solid tumors. Cell & Gene Therapy Insights. 2020;6(6):855-863. doi: 10.18609/cgti.2020.088.
-
Goff SL, Dudley M, Citrin DE, Somerville R, Wunderlich JR, Danforth DN, Zlott DA, Yang JC, Sherry RM, Kammula US, Klebanoff C, Hughes MS, Restifo NP, Kwong ML, Ilyas S, Klemen N, Payabyab E, Steinberg SM, White DE, Rosenberg SA. A randomized, prospective evaluation comparing intensity of lymphodepletion prior to adoptive transfer of tumor infiltrating lymphocytes for patients with metastatic melanoma. J Clin Oncol. 2016;34:3006-3006. doi: 10.1200/JCO.2016.34.15_suppl.3006.
-
Seitter SJ, Sherry RM, Yang JC, Robbins PF, Shindorf ML, Copeland AR, McGowan CT, Epstein M, Shelton TE, Langhan MM, Franco Z, Danforth DN, White DE, Rosenberg SA, Goff SL. Impact of Prior Treatment on the Efficacy of Adoptive Transfer of Tumor-Infiltrating Lymphocytes in Patients with Metastatic Melanoma. Clin Cancer Res. 2021;27(19):5289-5298. doi: 10.1158/1078-0432.CCR-21-1171.
Please note: This slide show is currently being updated to reflect the February 2024 FDA approval of this therapy. Please continue to explore the website, and check back for this updated slide show soon.
This slide show provides an overview of
Tumor Infiltrating Lymphocyte (
TIL)
Cell Therapy for
melanoma. TIL Cell Therapy is a
new type of immunotherapy that utilizes TILs to attack cancer cells. On February 16, 2024, the FDA approved AMTAGVI, the first cellular therapy indicated for the treatment of adult patients with cutaneous melanoma that is unable to be removed with surgery (unresectable) or has spread to other parts of the body (metastatic). AMTAGVI is a tumor-derived autologous T cell immunotherapy composed of a patient's own T cells, a type of cell that helps the immune system fight cancer. In this slide show, you will learn how TIL Cell Therapy is
prepared in the lab, how the
infusion works, its
effectiveness, possible
side effects, and its current use in
melanoma clinical trials. You may want to ask your doctor if TIL Cell Therapy could be an option for you.
-
Share with family and friends:
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about melanoma which will help other patients, caregivers and families.