Slide Show - Clinical Trials in Melanoma
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about melanoma which will help other patients, caregivers and families.



















Understanding Clinical Trials in Melanoma
*Please note: This slide show is not intended to be a substitute for professional medical advice. Always consult your doctor about any questions you may have regarding a medical condition.
What are melanoma clinical trials?
Clinical trials are research studies in which people volunteer to help test new ways to screen for or diagnose melanoma, new procedures, or new treatments.
What are melanoma clinical trials?
Clinical trials help researchers develop better tests and treatments. They also tell doctors about safety and side effects.
Most clinical trials in melanoma test new treatments. In this animation, we will focus on trials of new melanoma treatments.
How might I benefit from joining a melanoma clinical trial to test a new treatment?
By joining a clinical trial for a new melanoma treatment, you may be able to get the new treatment before it's available anywhere else.
You may receive:
By helping to find out how well a new treatment works, you may help other patients with melanoma.
Are there risks to being in a clinical trial?
Being in a clinical trial poses some risks:
If you decide to join a trial, but then change your mind, you can leave at any time, for any reason. This won't affect the care you receive from your healthcare team.
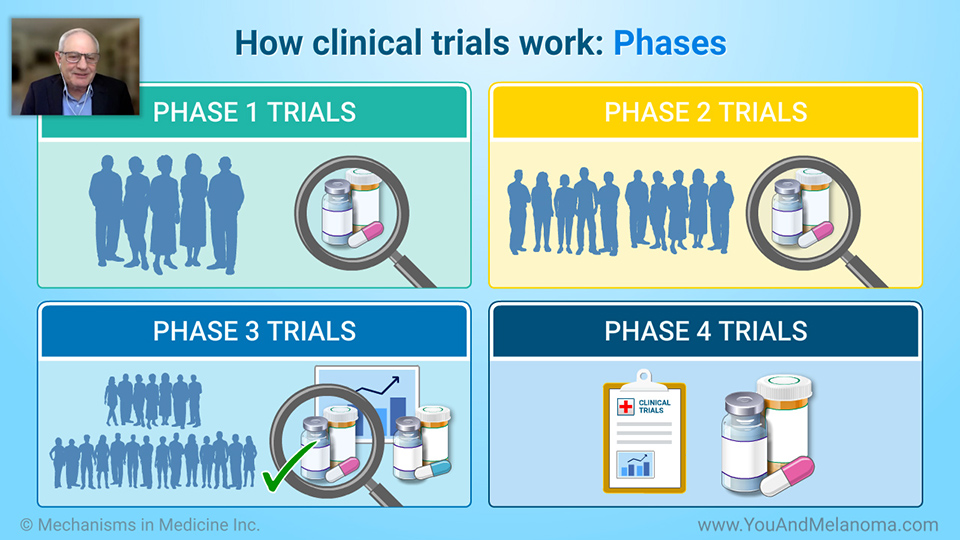
How clinical trials work: Phases
Clinical trials have four phases.
In Phase 1, a small group of volunteers tests the treatment to learn how safe it is, its side effects, and what is the optimal dose and schedule.
In Phase 2, a larger group of volunteers tests the treatment's safety and effect on the disease.
How clinical trials work: Phases
In Phase 3, many volunteers take the treatment to learn how well it works compared to current treatments.
If the treatment proves to be safe and effective, it can be approved for use outside of a clinical trial.
In Phase 4, researchers do further studies to learn how best to use the newly approved and marketed therapy.
Getting started in a clinical trial: Eligibility criteria
Most clinical trials have limits on who can take part, called eligibility criteria. These limits help ensure a margin of safety for those receiving the treatment.
Clinical trial terms: Informed consent
Here are some terms you may hear about clinical trials.
Informed consent is a process that helps you learn about a trial and decide if you want to join it.
Informed consent includes a document that explains the trial in basic language and how your rights will be protected. If you decide to join the trial, you'll be asked to sign this document.
Clinical trial terms: Placebo
A placebo is an inactive substance that looks just like the treatment being tested and is given in the same way.
Clinical trial terms: Placebo
In a cancer treatment trial, a placebo may be used to help researchers learn what effect a new treatment really has.
For example, a placebo may be used:
Clinical trial terms: Randomized and blinded
A randomized trial compares two or more treatments. To avoid bias, a computer allocates treatments randomly to trial participants prior to starting the study.
Clinical trial terms: Randomized and blinded
Blinding means that you, your doctors, or both, don't know which treatment you're receiving until the trial is unblinded. This is another step to avoid bias. You will be followed closely with appropriate exams and tests regardless of your treatment assignment.
Clinical trial terms: Protocol
A protocol is a detailed plan for how the trial will be conducted. Following the protocol helps ensure that the results are valid.
Questions to ask your healthcare team about a melanoma clinical trial
Here are some questions you may want to ask your healthcare team about a melanoma clinical trial:
Consider taking notes or bringing someone with you when you meet with your healthcare team to discuss these questions.
Is a melanoma clinical trial right for me?
Only you – together with your loved ones and your healthcare team – can decide if a clinical trial is right for you.
Is a melanoma clinical trial right for me?
Keep in mind that:
Be sure to discuss all your treatment options, including clinical trials, with your healthcare team.
References
Click here to take our SURVEY
Your feedback is important to us! We will use your feedback to develop future areas of content about melanoma which will help other patients, caregivers and families.





















This educational activity has been developed by the Melanoma Research Foundation (MRF), and Mechanisms in Medicine Inc.
This activity is supported by independent educational grants from Bristol-Myers Squibb, Foundation Medicine, Genentech, Iovance Biotherapeutics, Merck, Natera, and Novartis.







This website is part of the Animated Patient™ series developed by Mechanisms in Medicine Inc., to provide highly visual formats of learning for patients to improve their understanding, make informed decisions, and partner with their healthcare professionals for optimal outcomes.

